Have Anxiety About Your Joint Commission Audit? Achieve Compliant Logging and Consistent, Quality Disinfection - Part I

What's the problem?
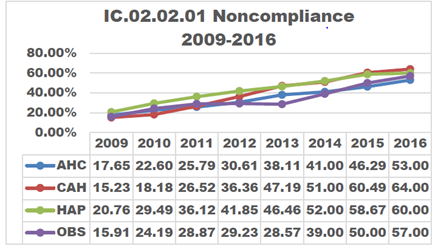
As the steps to reprocess medical devices using high-level disinfection increase and the population of patients imaged increases, non-compliance in the disinfection process increases proportionately.
In 2014, The Joint Commission (TJC) directed attention to improperly sterilized or high-level disinfected (HLD) equipment. Three years later in May of 2017, a follow-up article was released discussing the continuation and the increase of non-compliance. It stressed that in 2016, 74% of all immediate threats to life declarations were related to equipment improperly sterilized or high-level disinfected.1
A non-compliance issue can have negative consequences for a facility. The article also mentioned six ways non-compliance can affect a practice:1
- Placing patients at risk for contamination
- Causing potential outbreaks
- Potential loss of The Joint Commission accreditation
- Potential loss of Centers for Medicare and Medicaid Services (CMS) deeming status
- Bad publicity, lost business, and a damaged reputation
- Litigation
How is a facility non-compliant?
The article from the Joint Commission Resources called Solutions Part 2: How to Meet the Most Challenging Medical Records and Infection Control Standards reads, "Standardizing the use of high-level disinfectants and sterilization practices are critical for ensuring that medical equipment, devices, and supplies do not transmit infectious agents to patients."2
TJC noted the five most frequently noted areas for improvement:2
- Lack of having/using evidence-based guidelines
- Deficit in orientation of employees, training, and continued competencies
- Shortage in quality control and quality monitoring of processes related to ensuring concentration, exposure time, MRC testing, and exposure temperature for each cycle is accurate and documented/logged
- Participation and collaboration of practitioners and infection prevention in monitoring high-level disinfection
- Record keeping and inexplicable, non-standardized logs weaken the validation, monitoring, and accountability of a process
The absence of documentation on equipment makes it hard to track the apparatus used during an exam, which is important in the notification of an outbreak that needs to be conveyed to a patient
The Remedy to Customer Anxiety
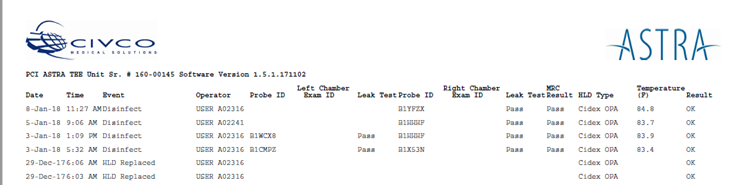
The ASTRA systems provide a simple way to show The Joint Commission auditor, a department's reprocessing method and process. ASTRA's customizable logging system is built into the device and automatically documents the following steps:
| • Date | • Probe ID | • HLD Type |
| • Time | • Rt/Lt Chamber Exam ID | • Temperature Monitoring |
| • Event | • Leak Test | • Result |
| • Operator | • MRC Test |
This automated disinfection process is designed to help with employee compliance as there is no need to "babysit the probe," which can take time away from patient care. It can, also, save on potential damage to the transducer due to over-soaking in the potent chemicals.
Human error is the enemy of compliance logging and consistent, quality probe disinfection. Understanding why logging is important is crucial for compliance. For instance, monitoring and documenting chemical temperature.
There are minimum requirements for how warm the disinfectant should be for effective disinfection. If the temperature isn't at the minimum degree recommended by the manufacturer, then the disinfectant isn't strong enough to clean the probe effectively. This is why The Joint Commission requires tracking the disinfectant temperature. If the chemical doesn't clean the probe, then the patient is not fully protected - which presents a cross-contamination risk.
Manually checking and logging the disinfectant temperature has a high potential for human error, as employees have routines and adding a step can lead to inconsistencies and can be difficult to enforce. Automated reprocessing and logging solves these issues. With ASTRA, even the easy-to-overlook items such as the air filter, leakage tester, and chemical expiration dates are tracked to make the process more efficient and effective.
Protecting patients, improving a department's process, and shielding medical facilities from the potential damage that outbreaks, loss of accreditation, bad publicity, and litigation can cause is the goal of CIVCO with the ASTRA TEE and VR. Contact CIVCO to learn more about how ASTRA can help improve your facility's probe disinfection process and achieve confident compliance. To get more information on CIVCO's ASTRA TEE and VR click here.

Part 2 of Have Anxiety About Your Joint Commission Audit? will highlight ASTRA customers and their personal experiences with ASTRA automatic high-level disinfection. Don't miss it!
References
- www.jointcommission.org/assets/1/23/qs_33a_2017.pdf
- "Solutions Part 2: How to Meet the Most Challenging Medical Records and Infection Control
- Standards." Joint Commission Resources Quality & Safety Network, 2014