High Level Disinfection Log and Temperature Guidelines

PCI Medical (CIVCO Medical Solutions) customers often ask us questions related to the temperature of their high-level disinfectants. Some questions are commonly asked about how to maintain the temperature of the high-level disinfectant, how to measure its temperature or how to record it. Let's address the questions surrounding this common topic, one by one.
At what temperature should I maintain the high-level disinfectant?
When disinfecting an instrument with an automated reprocessor, the device will heat the disinfectant to the required temperature. If manually disinfecting an instrument in a GUS Disinfection Soak Station, for example, the temperature depends on the chemical sterilant being used. Always refer to the disinfectant manufacturer's Instructions for Use.
- OPA disinfectants, such as CIDEX® OPA, recommend a minimum of 68°F.
- Hydrogen peroxide disinfectants, such as UltrOx™, recommend a minimum of 68°F
- Glutaraldehyde, such as CIDEX, recommend a minimum of 77°F.
 How do I achieve the required high-level disinfectant temperature?
How do I achieve the required high-level disinfectant temperature?
You can safely warm the high-level disinfectant to meet the minimum required temperature with a disinfectant warmer. The warmer is designed to fit our new 17" long containers which accommodate all vaginal/rectal ultrasound probes. Two models are available, depending on which GUS Disinfection Soak Station you operate. Another simple fix is to set the ambient temperature to 70°F if you're trying to achieve a minimum of 68°F.
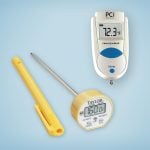 How do I measure the temperature of the high-level disinfectant?
How do I measure the temperature of the high-level disinfectant?
(Centers for Medicare & Medicaid Services) Inspectors check to see if instruments are disinfected at the appropriate temperature. CIVCO offers an infrared, instant-read thermometer.
What should I document during the high-level disinfection process?
The Joint Commission (TJC) surveyor will always look for documented evidence of quality processes. It is important to document the shelf life of the MRC (Minimum Recommended Concentration) test strips and the high-level disinfectant. They will look to see if the high-level disinfectant has been tested and documented for the appropriate concentration. TJC could also ask for the QC test strip data.
Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities. ANSI/AAMI ST79: 2010/A2: 2011.
The following is taken from a sample CMS survey form:
The chemicals used for high-level disinfection are:
- Prepared according to the manufacturer's instructions
- Tested for appropriate concentration according to the manufacturer's instructions
- Replaced according to manufacturer's instructions.
- Documented to have been prepared & replaced according to manufacturer's instructions
The instruments that require high-level disinfection are:
- Disinfected for the appropriate length of time as specified by the manufacturer's instructions
- Disinfected at the appropriate temperature as specified by the manufacturer's instructions or evidence-based guidelines
(Sample CMS Survey for ASC, Infection Control Surveyor Worksheet, Exhibit 351)
When should I test the concentration level of the disinfectant?
Chemical test strips, or biological indicators, are required to test the concentration level of the disinfectant, whether you are disinfecting an instrument manually or in automated disinfector. They will determine whether a reusable disinfectant still meets the minimum effective concentration levels, known as MEC (Minimum Effective Concentration) or MRC (Minimum Recommended Concentration).
"The frequency of testing should be based on how frequently the solutions are used (e.g. used daily, test daily; used weekly, test before use; used 30 times per day, test each tenth use). The results of test strip monitoring should be documented in a written log. The bottle of the test strip should be dated when opened and used for the period of time indicated on the bottle."
Rutala, Sterilization, High-Level Disinfection, and Environmental Cleaning. Pg 55.
The manufacturers of CIDEX OPA recommend using their test strips prior to each cycle. Like other high-level disinfectant manufacturers, they recommend discarding the disinfectant even if the disinfectant has met the MRC test, but has gone beyond the reuse date.
It is important to store the chemical test strips or biological indicators according to their instructions to prevent them from drying out and be sure to check that the bottles are properly sealed and closed.
How do I safely neutralize the high-level disinfectant?
When disposing of used disinfectant, use PCI Medical's Glute-Out Neutralizer to deactivate the chemical. A common misconception is that high-level disinfectant is no longer toxic if it has failed its MRC test or gone beyond the reuse date. Read our whitepaper on the Safe Neutralization of High-Level Disinfectants.




